
27 February 2023
The JCA will be mandatory for manufacturers to participate in and prepare for submission. The considerations from the JCA will be non-binding for each member state, however, they are expected to include the JCA report in their HTA considerations.
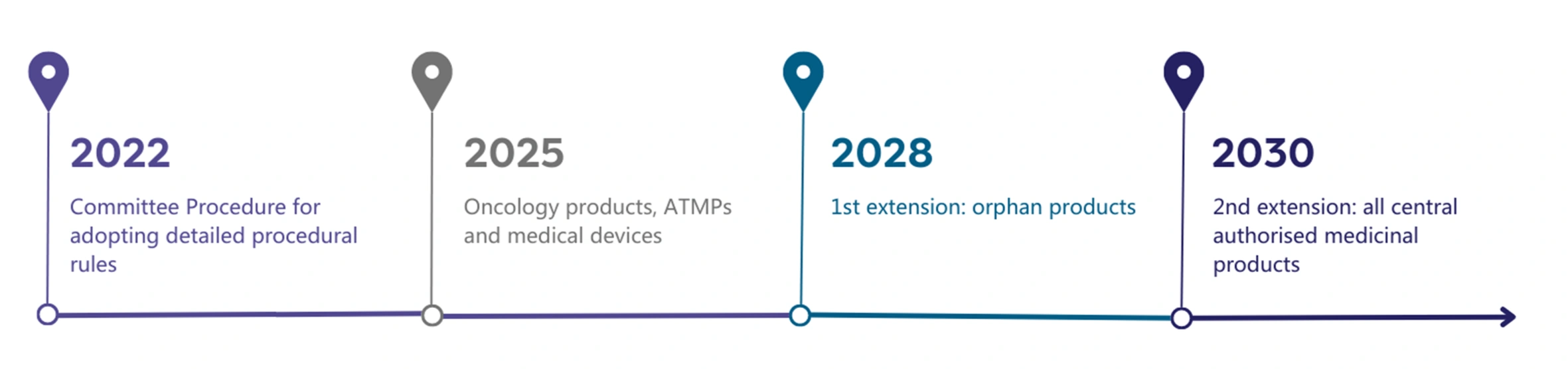
Figure 1: Timeline for the implementation of the EU HTA process.
What could the JCA mean for orphan drugs?
The JCA is anticipated to provide clear and coherent clinical evidence requirements for orphan drugs. This introduces the opportunity to have timely decision-making and faster access in the EU, as the joint system removes the differences seen in national Health Technology Assessment (HTA) bodies evidence requirements.2 However, it is anticipated that some member states such as Germany and France may require additional clinical data following the JCA to make an informed decision, this could further delay access in these states compared with rest of EU. More so, negative considerations from the JCA could further delay access to the orphan drug and could therefore be damaging to the reputation of the drug at the EU level.
A key advantage of the JCA for orphan drugs may be the increase in transparency and engagement of EU patients within the HTA process.2 Patient advocacy is considered to be very important for orphan drugs as the diseases treated impact small patient populations who would have an instrumental input in the assessment process.
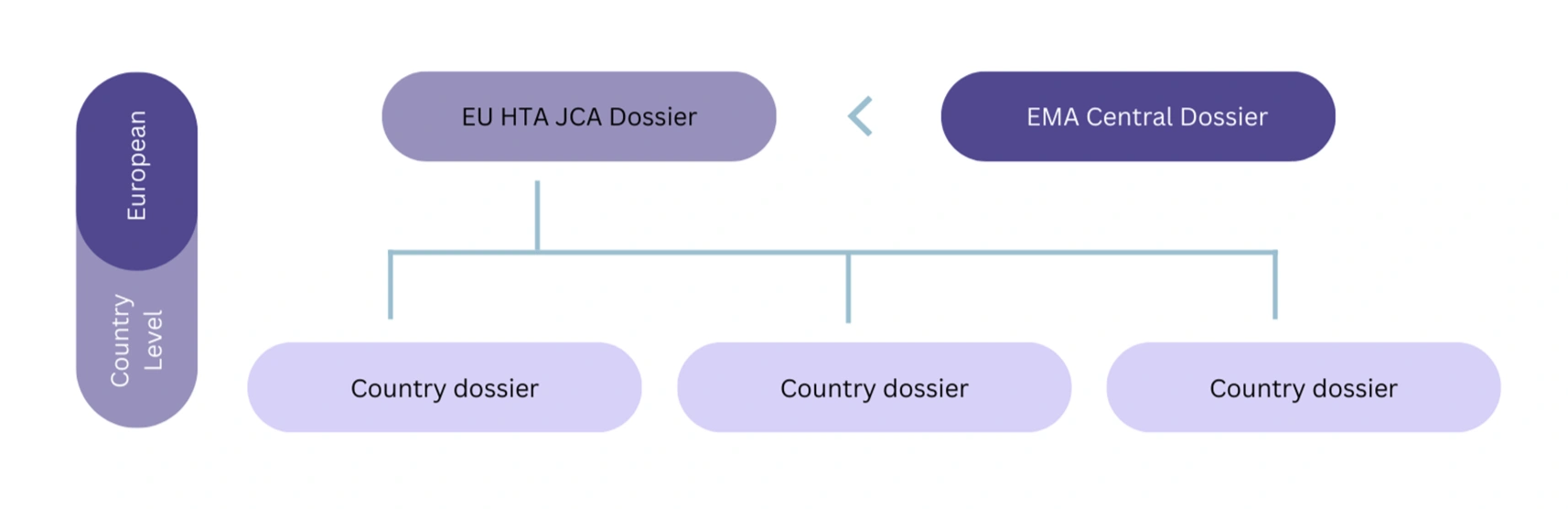
Figure 2: Schematic to show potential additional dossier requirements after the JCA, at the country level.
What are EU member states views on the JCA?
The joint system has come into scrutiny from some member states. In Germany, the vfa (German association of research-based pharmaceutical companies) and BPI published a joint position paper, stating their concerns over further fragmentation and misalignment due to the EU HTA. The vfa has argued that the proposed EU HTA should not be a amalgamation of national process but rather a unified new approach.
Germany is the only EU market with special consideration for orphan drugs, where orphan drugs can undergo abbreviated assessment in which a comparative data is not required and lowest added benefit rating attainable is ‘not quantifiable added benefit’. However, a full assessment is triggered once the €30 million annual sales threshold is reached. Another concern raised by the vfa is the lack of consideration the EU HTA process has put in place for orphan drugs or ATMPS, which may lead to these drugs not coming into the EU.
According to GPI sources, member states such as Italy are already planning on implementing processes next year to accommodate for the JCA.4 As the vision for the joint system is becoming a reality, the acceptance or disagreements between the member states will become more apparent over the next few years.
The JCA is an evolving process, and GPI will be monitoring any changes from the EUnetHTA as they arise. In the meantime, if you would like a quick demonstration of GPI’s analytics platform 2.0, using the leading biopharma drug pricing submit in the form below.
