23 November 2022
Minimising the risk of non-compliance for pharma companies
As the need for greater transparency of drug prices in the United States continues, more states move to introduce legislation focusing on state-level visibility or control of drug price increases and cost of newly launched medicines. The laws are complex, vary from state to state, and are dynamically evolving, with many states penalising non-compliance. It is, therefore, critical for pharmaceutical companies to understand legislative requirements, keep abreast of changes and comply with all reporting requests accurately to prevent costly retributions.
Because of the differing requirements from state to state, it is increasingly difficult for the pharma companies to keep up with the rules, deadlines and/or any changes in the requirements. Many rely on traditional methods, meaning information can be missed, work duplicated and therefore are at risk of non-compliance.
Vermont passed the first state drug price transparency law in 2016 by requiring annual reporting of selected medicines with significant spend. Today, more than a dozen states have passed similar, and in some cases wider, price transparency laws. Many of the state price transparency regulation requirements fall into one of the following categories:
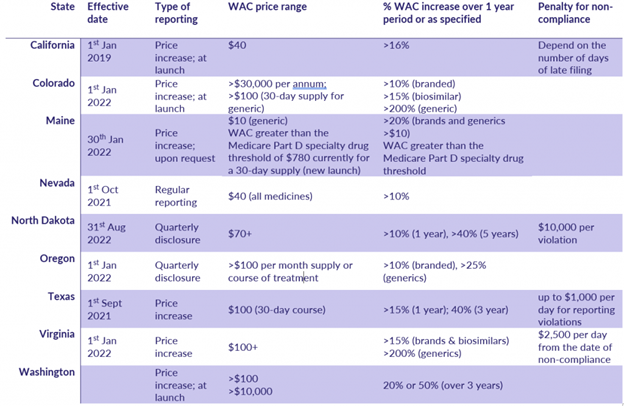
- Notification of price increases: Companies that plan to increase the wholesale acquisition cost (WAC) above a certain threshold or at a rate over a certain timeframe are required to notify legislative state or federal bodies see fig 1.
- Price reporting: Companies that sell drugs in certain states are required to periodically report pricing to the state or upon the launch of a new medicine that has a price that exceeds a specific threshold (for example, WAC at launch exceeds the Medicare Part D specialty drug threshold) or requires companies to disclose WAC or the average wholesale price (AWP) of a drug to the state or health care providers.
- Budget control: The state may request information on medicines that have a substantial cost to the state, that are critical to public health or may have significant budget impact upon launch.
Speak to us about Price Transparency Reporting
Failing to comply with price transparency reporting laws can result in substantial penalties in some states. Nevada’s governing body issued penalties of around $17.4 million against non-compliant companies in 2019 and California levied $28 million in fines for reporting violations. Despite the costly penalties for non-compliance or errors, there are a number of challenges in ensuring the new reporting requirements are fully met:
Variable laws
State price transparency reporting legislation is inconsistent across states and requires careful analysis of each requirement to understand the intricacies for compliant reporting. See fig. 1 for example of recent state reporting needs.
GPI’s R&D efforts are focused on understanding criteria for successful asset selection, market access prediction and methods for operationalising market access strategy.
1 – Cost calculation complexities
Translating WAC price per unit to a ‘course’ of treatment such as the 30-day cost is relatively straight forward for pill or capsule formulations but is challenging to decipher when calculating costs for intravenous or topical formulations particularly where the treatment regimen itself is complex.
2 – Dynamic and evolving policy across multiple states
Reporting requirements are complex, rapidly evolving, and often include penalties for noncompliance and resource needs for company are high and time consuming to actively monitor newly enacted or enhanced legislation and understand the details of each reporting requirement. New pending price transparency legislation in Pennsylvania, Iowa, Massachusetts, New York, and North Carolina are expected to be published soon.
3 – Internal cross-functional expertise:
Sourcing information internally may be challenging due to the cross-functional complexity that affects multiple departments throughout an organization, such as legal, compliance, government pricing, government affairs, and market access. A lack of interdepartmental understanding of the pricing calculation complexities and communication may pose challenges implementing and maintaining consistent processes and documentation that is needed to report information in a consistent and timely fashion.
4 – Impact of public disclosures.
Manufacturers may need to address the complexities that could result from reported prices being available to the public and should assess the legal implications of what to report. The specific information that manufacturers are required to report varies and often touches upon sensitive information, ranging from the profit earned for a specific drug to the direct costs incurred to manufacture the drug. This may create competitive challenges and public relations issues or lead to information being misinterpreted.
The importance of automated data and accurate reporting
Ensuring state specific timelines are met and providing the right data is key for ensuring requirements adhered to. Automating that data is important to ensure timelines are met and no work is duplicated or missed.
Global Pricing Innovations (GPI) partners with manufacturers to stay ahead of evolving policies, navigate complex interpretation and support timely and accurate reporting of price and 30-days costs with clear supporting assumptions and methodology. We partner with companies to provide best-in-class support for ongoing reporting including the implementation of state drug price transparency system and tools that stores pricing information with the ability to run pricing scenarios and generate reports at state level.
Reduce risk of inaccurate or delayed state price transparency reporting:
- Instant access to accurate drug prices and 30-day cost data
- Ensure timelines are met and minimise risk of non-compliance
- API’s connecting data to 3rd party applications
Don’t forget to watch our webinar ‘The importance of automating treatment data’ to see how we’re helping company navigate the complexities of state price transparency requirements.
GPIs internal team of domain experts are well-versed in regulatory and price reporting internationally including interpreting the complexities of converting unit price to 30-day cost for complex formulations. Leveraging our team of pricing analysts and pharmacists, we give companies the help they need by continually monitoring current state drug price reporting requirements and confidence in accurate understanding and interpretation of label information. We provide readily accessible data covering state transparency pricing laws and provide timely updates to ensure that all reporting obligations are met within the required timeframes.
