28 January 2022
On 20 January 2022, the National Institute of Care and Excellence (NICE) announced board approval of its long-anticipated reforms on the health technology assessment (HTA) processes and methods. These changes coming into effect next month are expected to have a significant impact on access to innovative therapies.
Challenges with the previous pricing scheme, Pharmaceutical Pricing Regulation Scheme (PPRS), in the United Kingdom (UK)
In 2019, the UK PPRS was replaced by the Voluntary Scheme for Branded Medicines Pricing and Access (VPAS). The PPRS had been criticised for creating barriers for patient access to innovative medicines, such as personalised medicines and cell therapies. The VPAS set out goals to accelerate the NICE appraisal process for these innovative therapies by simplifying the HTA process, improving uptake of approved medicines and speeding up access for National Health System (NHS) patients.
As a result, NICE committed to a methods review in 2019 to achieve the targets set in the VPAS. The long-anticipated changes to the HTA review process have now received final clearance for implementation.
Market access delays for innovative therapies in the UK
To further understand the market access environment in England, Global Pricing Innovations (GPI) compared the time to reimbursement of highly innovative therapies (Zolgensma, Spinraza, Luxturna and Kymriah) with Italy and France. We have defined time to reimbursement as the time between marketing authorisation until the final HTA decision date.
Since 2017, all four drugs have been granted marketing authorisation by the European Medicines Agency (EMA) for Spinal Muscular Atrophy (SMA), Retinal Dystrophy and Acute Lymphoblastic Leukaemia (ALL). However, the rate at which these therapies were reimbursed varies largely across the markets. On average, it took 410, 467 and 175 days for time to reimbursement for England, Italy and France, respectively.
In England, reimbursement of innovative therapies has taken almost double the amount of time compared with France, with French reimbursement times consistently shorter. Italy presents greater variation in timelines, and trails behind England on average in our sample. In addition, in our sample England presents the longest time to reimbursement, with 785 days seen for the reimbursement of Spinraza. The data suggests that there is a need to accelerate access for innovative therapies in England; the newly introduced NICE reforms have the potential to address possible inefficiencies and reduce the time to reimbursement.
Time to Reimbursement* of Innovative Therapies
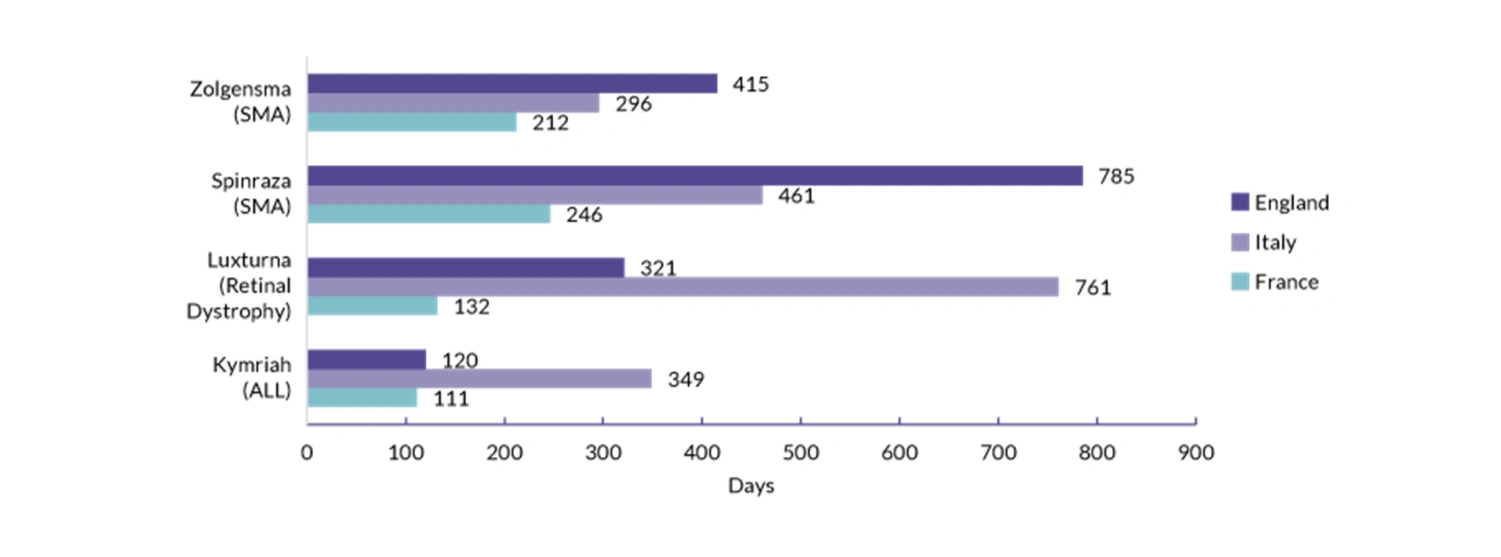
Notes: Time to reimbursement is defined as the number of days from the date of marketing authorisation to the HTA decision.
Source: GPI
Impact of NICE reforms on uptake of innovative therapies
The long-anticipated changes to the NICE HTA review process will likely impact the market access environment for innovation in the UK. The key elements worth highlighting are as below, the detailed report can be found here.
- Introduction of a severity modifier, which will provide a higher weighting for more severe conditions and replace the end-of-life modifier
- Introduction of an uncertainty modifier to manage uncertainty in the appraisals, by assessing risks to NHS patients without preventing access to valuable innovations
- Greater acceptance of real-world evidence (RWE) data to support the evidence package of innovate treatments
- A refined routing criteria for therapies in which NICE will evaluate under the Highly Specialised Technologies (HST) programme
- Earlier engagements for commercial or managed access scheme proposals
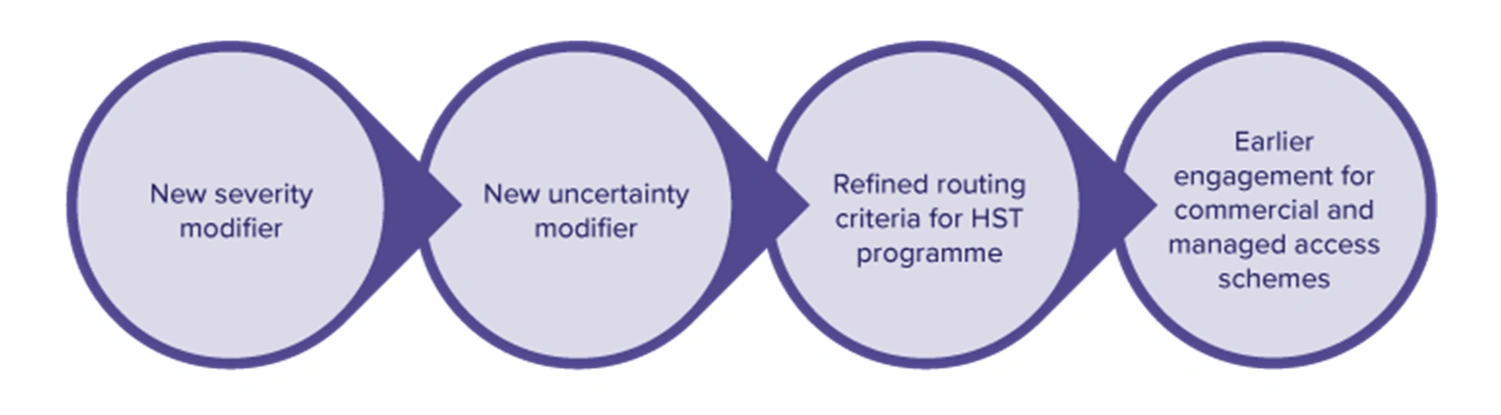
The addition of the severity and uncertainty modifiers aims to provide a more equitable access for therapies targeting severe and rare conditions, where robust data generation is highly challenging. Earlier engagements for commercial or managed access schemes and greater acceptance of RWE provide patients with the option of receiving treatments earlier while more evidence is being collected. The set out clear vision and reduction in routing criteria (from seven to four elements) for technologies eligible for HST programme will provide greater access for specialised therapies targeting very rare diseases.
The NICE changes coming into effect February 2022 aim to increase flexibility in its HTA process for innovative treatments, enabling a quicker and fairer access for NHS patients. The reforms will also present a greater opportunity for the UK to put itself at the forefront of innovation, providing an attractive market access environment for newly launched innovative treatments. However, the significance of these reforms in shortening reimbursement timelines for newly innovative therapies will only become evident with time.
Have a question for our experts?
Submit your query below, and one of our experts will contact you.
