22 January 2025
Background and Objectives
In Europe, orphan designation status plays a vital role in encouraging the development of treatments for rare diseases.
This special status grants valuable incentives—most notably, a 10-year market exclusivity period—designed to attract innovation and investment in life-saving therapies for rare conditions. But to receive this designation, a treatment must meet three essential criteria:
- The medicine must address a life-threatening or chronically debilitating disease.
- The prevalence of the disease in the EU (European Union) must not exceed 5 in 10,000 people, or the medicine must be unlikely to generate sufficient returns to justify its development.
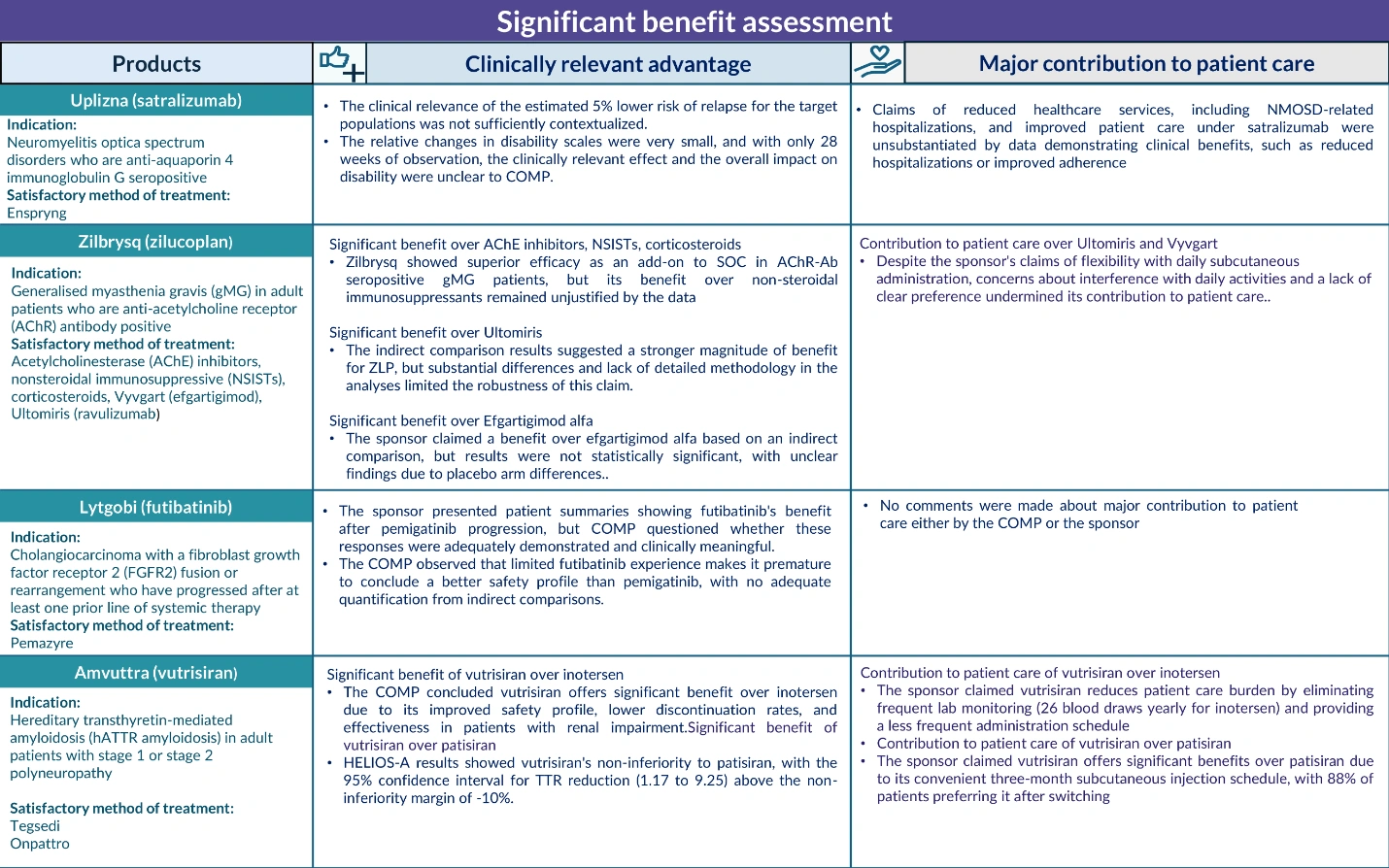
- There must be no satisfactory methods of diagnosis, prevention, or treatment for the condition—or, if such methods exist, the new medicine must demonstrate a “significant benefit” to patients. This is typically assessed by measuring its clinical advantage or its major contribution to patient care1.
When it’s time to apply for marketing authorization, sponsors must reapply to maintain this orphan designation status. This step ensures that the product still meets the orphan criteria before the Committee for Human Medicinal Products (CHMP) of the European Medicines Agency (EMA) grants market approval.
At this stage, if there’s already an authorized treatment available, sponsors must prove that their product offers a significant benefit over existing therapies. Importantly, the data required for maintaining orphan status at this point are more comprehensive than those for the initial designation2. If similar treatments exist, an evaluation of “orphan similarity” may also be necessary1.
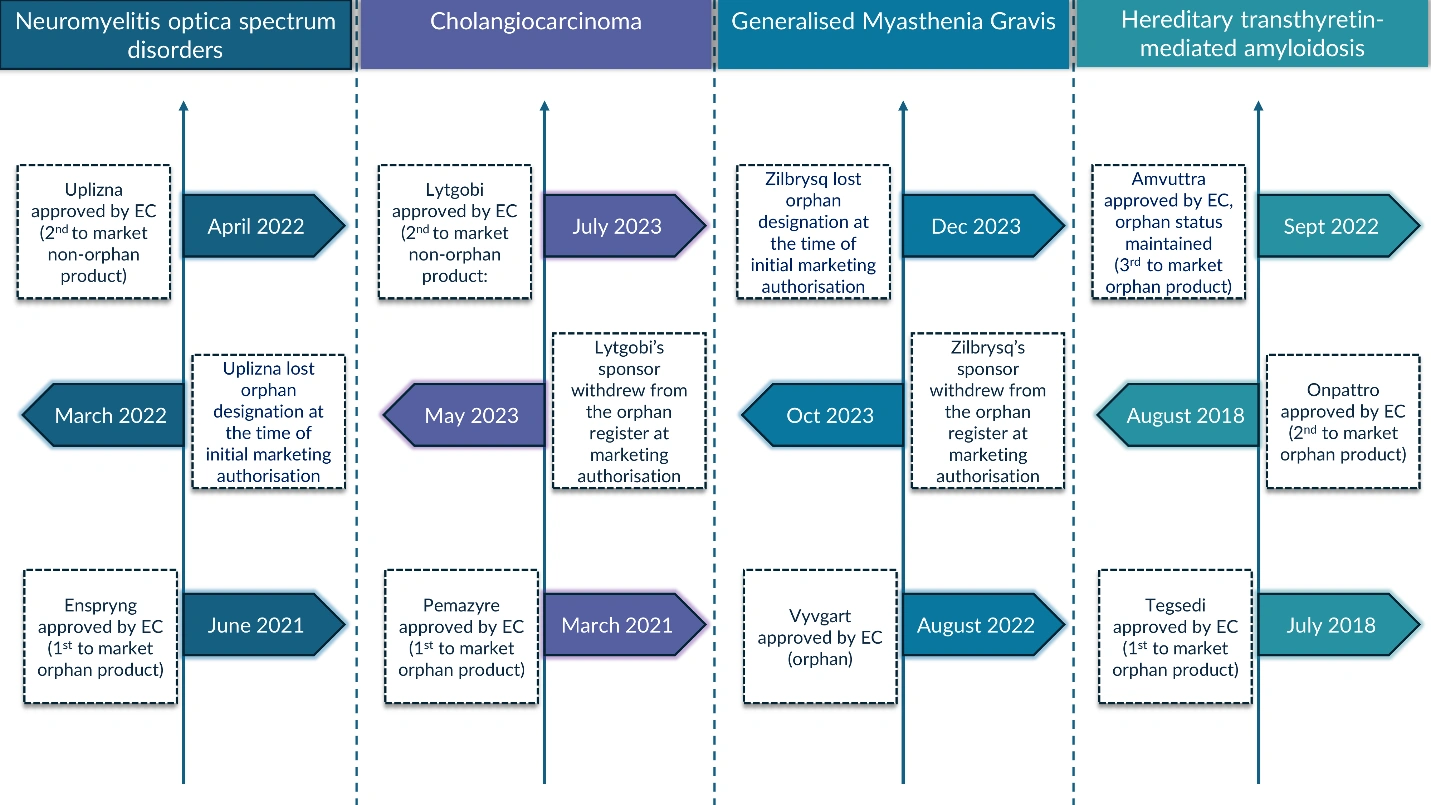
While this framework encourages first-of-its-kind treatments, it can inadvertently create obstacles for subsequent medicines aiming for orphan designation in the same indication.
The presence of a first-to-market orphan product—particularly one that has achieved meaningful outcomes in the indication—can complicate the pathway for second- and third-to-market products. Factors such as the timing of approval and the need to demonstrate significant benefit over existing options can impact the success of subsequent orphan designations.
In this research, we explore how first-to-market orphan products influence the orphan designation status of follow-up treatments within the same orphan indication.