
Overcome barriers
Inform strategy and maximise asset value by ensuring adherence with regulatory requirements across all aspects of market access and pricing. Stay updated with changing regulations across each market and rapidly adapt strategies and pricing to remain compliant and competitive.

Accelerate your
time to market

Find regulatory data quickly
Access our platform for regulatory data: country regulatory bodies, regulatory approval data, approved indication and dosing.

Stay informed
Leverage inbuilt tools to track price changes stemming from major regulatory events. Track International Reference Price (IRP) policies & the impact of regulatory changes, & make crucial strategic or price adjustments.

Be compliant
Maintain compliance with national and international pricing policies by accessing official labelling and regulatory requirements.

Streamline approval
Understand the HTA processes of each market and align submissions. Ensure you understand and meet evidence expectations of each market to optimise reimbursement potential.

Data you need,
available immediately

See competitor intelligence
Compare data from HTA reports. See what indications were reimbursed and the reasons that supported the decisions.
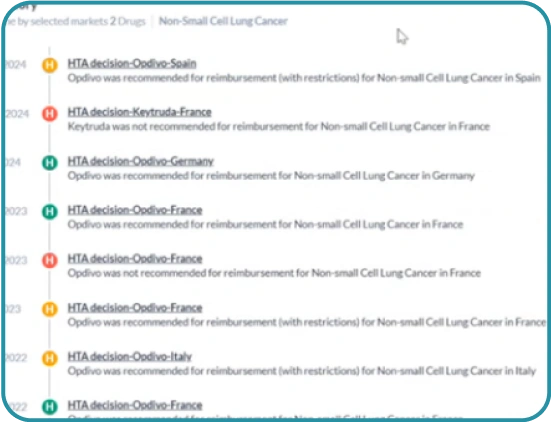
Align your timeline
See a full timeline of events for competitor products – examine their impact and time-to-reimbursement.

What makes GPI unique

We’ve already done the research
Our platform contains the essential data you need. Expert-validated IRP rules, regularly updated and clearly presented for each market. Summary of key country systems including regulatory and pricing policies.

GPI are market access specialists
Our qualified pharmaceutical market access consultants can help you overcome regulatory hurdles. With vital recommendations, from product due diligence, through to optimisation, we can help you to accelerate your time to market. Just ask.

Talk to an expert today about your market
access challenges
Our qualified market experts can help you optimise your plan.


